MEMED BV GENERAL
What are the three biomarkers in the MeMed BV® test?
MeMed BV computationally integrates the levels of three host immune proteins –TNF-related apoptosis-inducing ligand (TRAIL), Interferon gamma-induced protein-10 (IP-10) and C-reactive Protein (CRP) into a numeric score that falls within discrete interpretation bins based on the increasing likelihood of bacterial infection.
Where are MeMed BV® and MeMed Key® available?
MeMed BV and MeMed Key are FDA, CE-IVD and AMAR cleared and available in the US, EU, and Israel.
What sample type is required for the MeMed BV® test?
The MeMed BV® from serum test requires 100 µL collected using a Serum Separator Tube (SST) from a venous blood draw. MeMed BV from whole blood* test requires 150µl collected using EDTA (lavender top) tube from a venous blood draw.
*MeMed BV on venous whole blood is 510K cleared. Currently available only for the US market.
What are the indications for MeMed BV®?
US: The MeMed BV® test is an automated semi-quantitative immunoassay that measures three non-microbial (host) proteins (TRAIL, IP-10, and CRP) in adult and pediatric serum and venous whole blood samples and is intended for use in conjunction with clinical assessments and other laboratory findings as an aid to differentiate bacterial from viral infection. MeMed BV is indicated for use in patients presenting to the emergency department or urgent care center and with samples collected at hospital admission from patients with suspected acute bacterial or viral infection, who have had symptoms for less than seven days. The MeMed BV test generates a numeric score that falls within discrete interpretation bins based on the increasing likelihood of bacterial infection.
*MeMed BV on venous whole blood is 510K cleared. Currently available only for the US market.
EU and Israel: The MeMed BV test is an automated semi-quantitative immunoassay for professional clinical use only that measures three non-microbial (host) proteins (TRAIL, IP-10, and CRP) in adult and pediatric serum samples. The Test is intended for use in conjunction with clinical assessments and other laboratory findings as an aid to differentiate bacterial from viral infection. The Test is indicated for use in patients presenting to the emergency department, urgent care center and inpatients with suspected acute bacterial or viral infection. The MeMed BV test generates a numeric score that falls within discrete interpretation bins based on the increasing likelihood of bacterial infection. The MeMed BV test is intended for in vitro diagnostic use only.
What are the limitations of use for MeMed BV®?
US: The test is indicated for patients presenting to the emergency department or urgent care center and with samples collected at hospital admission from patients who have exhibited symptoms of acute infectious disease for less than or equal to 7 days and experienced fever within the last 7 days.
The overall prevalence of bacterial infection in a given population may affect assay interpretation.
The test has not been studied in patients under 90 days old, and patients with one or more of the following conditions:
- Suspicion and/or confirmed diagnosis of infectious gastroenteritis/colitis;
- Active inflammatory disease;
- Congenital or acquired immune deficiency;
- Chronic fungal or parasitic infection;
- Infection with human immunodeficiency virus (HIV);
- Hepatitis B virus (HBV);
- Hepatitis C virus (HCV);
- Infection with active tuberculosis (TB);
- Significant trauma or burns in the last 7 days;
- Patients that have undergone major surgery in the last 7 days.
- Pregnant women
- Active malignancy
- Whole blood only – patients with hematocrit levels under 30% or over 50%
The test is not intended as a standalone diagnostic test, is not intended to identify specific pathogens or predict disease course and is not intended to distinguish between infectious and non-infectious etiologies.
EU & Israel: The test is indicated for hospital admitted patients, and patients presenting to the emergency department, urgent care center who have exhibited symptoms of acute infectious disease for less than 7 days and experienced fever within the last 7 days.
The overall prevalence of bacterial infection in a given population may affect assay interpretation.
The test has not been studied in patients under 90 days old, and patients with one or more of the following conditions:
- Suspicion and/or confirmed diagnosis of infectious gastroenteritis/colitis;
- Active inflammatory disease;
- Congenital or acquired immune deficiency;
- Chronic fungal or parasitic infection;
- Infection with human immunodeficiency virus (HIV);
- Hepatitis B virus (HBV);
- Hepatitis C virus (HCV);
- Infection with active tuberculosis (TB);
- Significant trauma or burns in the last 7 days;
- Patients that have undergone major surgery in the last 7 days.
- Pregnant women
- Active malignancy
The test is not intended as a standalone diagnostic test, is not intended to identify specific pathogens or predict disease course and is not intended to distinguish between infectious and non-infectious etiologies.
How does MeMed BV® compare to clinical and laboratory parameters?
MeMed compared the performance of the MeMed BV test in distinguishing bacterial from viral infection to that of commonly used clinical and laboratory parameters. Each parameter was measured solely and in combinations: best performing pair (ANC and Lymph %), triplet (ANC, Lymph % and Pulse), and quadruplets (ANC, Lymph %, Pulse, Mono %).1
Of the multiple comparisons, the MeMed BV test performed significantly better (P<10-15).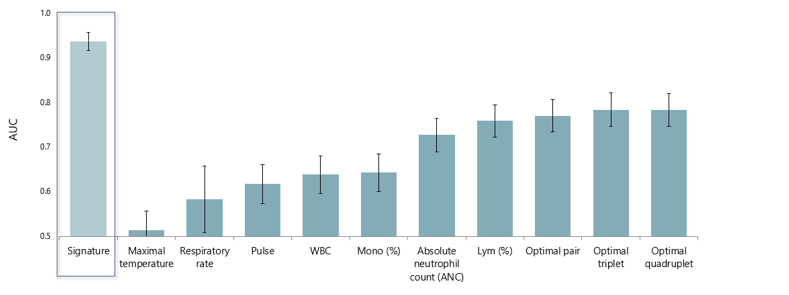
- Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PloS One. 2015 Mar 18;10(3):e0120012.
Can I use MeMed BV® on neonates?
MeMed BV has not been studied in patients under 90 days old.
Can I use MeMed BV® alone to make clinical decisions?
MeMed BV is indicated for use in conjunction with clinical assessments and other laboratory findings as an aid to differentiate bacterial from viral infections. By providing a rapid, accurate result based on the host response to determine likelihood of bacterial or viral infection, MeMed BV helps physicians to make faster, more informed diagnostic decisions.
How does MeMed BV® compare to Procalcitonin (PCT)?
Procalcitonin (PCT) is an amino acid precursor of calcitonin and can be applied to aid in differential diagnosis decisions for patients with lower-respiratory tract infections (LRTI) in an inpatient setting or Emergency Department and on antibiotic discontinuation for patients with sepsis.
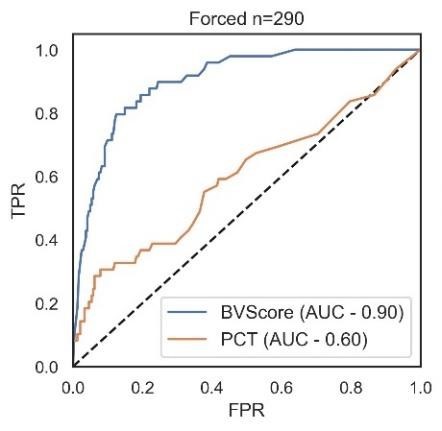
Figure from Oral Presentation at ACEP 2021 by Richard Rothman, MD PhD. These data are the findings of a sub-analysis of the Apollo Study (NCT04690569; data on file). The sub-analysis focused on patients presenting to the emergency department, n = 290. TPR, true positive rate; FPR, false positive rate.
MeMed BV has several advantages over PCT:
- MeMed BV compares favorably on determining bacterial versus viral infection (see Figure)
- Multiple studies establish MeMed BV’s diagnostic accuracy not only in adults but, unlike PCT.1-5
- MeMed BV’s intended use is applicable for patients with suspected bacterial or viral infection and not restricted to only patients with LRTI or sepsis like PCT
- MeMed BV is cleared for use not only in the emergency department and during patient admission but, unlike PCT, also at urgent care centers
- Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PloS One. 2015 Mar 18;10(3):e0120012.
- Ashkenazi-Hoffnung L, Oved K, Navon R, Friedman T, Boico O, Paz M, et al. A host-protein signature is superior to other biomarkers for differentiating between bacterial and viral disease in patients with respiratory infection and fever without source: A prospective observational study. Eur J Clin Microbiol Infect Dis. 2018 Jul;37(7):1361-71.
- Stein M, Lipman-Arens S, Oved K, Cohen A, Bamberger E, Navon R, et al. A novel host-protein assay outperforms routine parameters for distinguishing between bacterial and viral lower respiratory tract infections. Diagn Microbiol Infect Dis. 2018 Mar 1;90(3):206-13.
- Mor M, Paz M, Amir L, Levy I, Scheuerman O, Livni G, et al. Bacterial vs viral etiology of fever: A prospective study of a host score for supporting etiologic accuracy of emergency department physicians. PLoS One. 2023 Jan 30;18(1):e0281018.
- Papan C, Argentiero A, Porwoll M, Hakim U, Farinelli E, Testa I, et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: A prospective, multicentre cohort study. Clin Microbiol Infect. 2022 May 1;28(5):723-30.
How does MeMed BV® address emerging pathogens?
MeMed BV is pathogen agnostic, in that it detects our immune response to the pathogen and does not detect the pathogen itself. The performance of MeMed BV in differentiating between bacterial and viral infection has been validated in multiple prospective, multi-center, double blinded, independent studies and real-world clinical use on thousands of patients with reproducible results. The studies included patients infected with many different bacterial or viral pathogens, and multiple strains, supporting that MeMed BV should also perform well for emerging pathogens.
How early in infection can MeMed BV® be used?
MeMed BV is intended for use in patients who have had symptoms of acute infection for up to 7 days, with one of the symptoms being fever.
The performance of MeMed BV in differentiating between bacterial and viral infection in such patients has been established irrespective of time from symptom onset.1-3
- Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PloS one. 2015 Mar 18;10(3):e0120012.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120012 - van Houten CB, de Groot JA, Klein A, Srugo I, Chistyakov I, de Waal W, et al. A host-protein based assay to differentiate between bacterial and viral infections in preschool children (OPPORTUNITY): A double-blind, multicentre, validation study. Lancet Infect Dis. 2017 Apr 1;17(4):431-40.
https://pubmed.ncbi.nlm.nih.gov/28012942/ - Papan C, Argentiero A, Porwoll M, Hakim U, Farinelli E, Testa I, et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: A prospective, multicentre cohort study. Clin Microbiol Infect. 2022 May 1;28(5):723-30. https://www.sciencedirect.com/science/article/pii/S1198743X21006212
We already use respiratory pathogen panels for pathogen detection, what added value does MeMed BV® provide?
In many cases, it is not possible to detect a pathogen using a respiratory pathogen panel. This can be because the pathogen is inaccessible (for example, located in the lower respiratory tract) or not targeted by the pathogen-specific panel (for example, an emerging strain). In addition, pathogen detection is intrinsically constrained in that detection does not necessarily equate with symptoms causation (e.g. colonizers).
One added value of MeMed BV is that the test does not require access to the pathogen, as it is based on immune proteins that circulate in the blood. Another added value of MeMed BV is that it interprets our immune response to the pathogen, and so the test result is not affected by non-invasive colonizers that do not trigger our immune system.
If a pathogen is already detected, is there added value to the MeMed BV® test?
Pathogens detected by various technologies (e.g., culture, or rapid PCR) may be merely “bystanders” (colonizers) and not the underlying cause of a patient’s current illness. Examples include:
Throat Culture: A positive Group A Streptococcus culture (GAS) confirms the presence of GAS, but it is unable to differentiate between colonization and the etiology of the acute illness.
PCR: The detection of a viral respiratory pathogen by PCR does not exclude the possibility of bacterial co-infection.
An added value of MeMed BV is that the result can help the physician decide if the detected pathogen is likely the clinically relevant pathogen that is causing the illness.
Can MeMed BV® detect co-infections?
MeMed BV provides a bacterial score when there is a bacterial-induced host immune response.
This may occur in either of the following circumstances:
- Sole bacterial infection
- Concomitant viral and bacterial infections
Multiple studies have shown that MeMed BV accurately distinguishes between sole viral and viral/bacterial co-infection helping physicians to make faster, more informed diagnostic decisions.
MEMED BV PERFORMANCE
What’s the MeMed BV ROC-AUC (receiver operating characteristic - area under the curve) and how does it relate to sensitivity and specificity?
ROC-AUC is a threshold free approach to compare different biomarkers. From a statistics standpoint, AUC integrates the sensitivity and specificity at each threshold into a single number.1 Based on secondary endpoint analysis in the Apollo Clinical Study (NCT04690569), MeMed BV demonstrates an AUC of 97%.
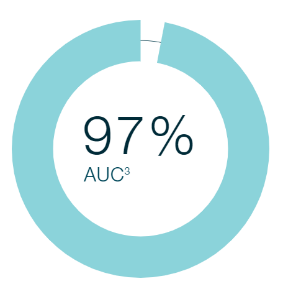
- MeMed data on file. Apollo Clinical Study (NCT04690569) conducted to establish the diagnostic performance of the MeMed BV test for differentiating bacterial from viral infection in patients with suspected acute bacterial or viral infection.
What clinical data do you have to back up MeMed BV® performance?
MeMed BV performance has been confirmed in blinded validation as well as in real-world evidence, altogether used in more than 20,000 patients. Conducted in in Europe, Israel and the United States, these studies have consistently demonstrated high diagnostic accuracy across different clinical settings, age groups, and patients with different clinical syndromes.1-6
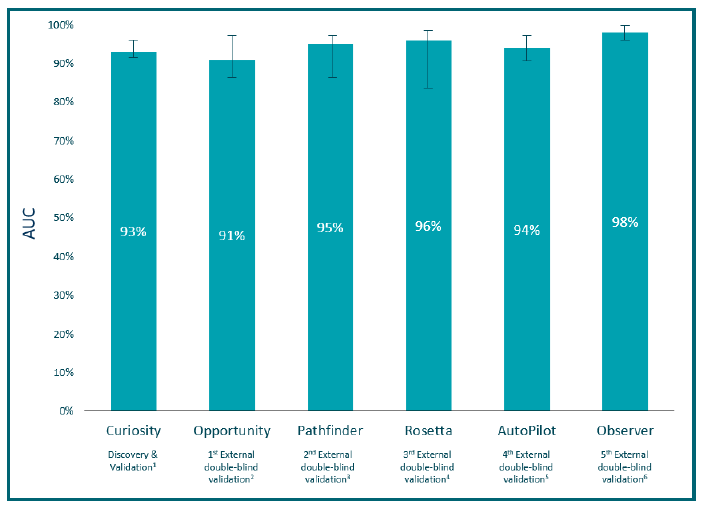
- Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PloS One. 2015 Mar 18;10(3):e0120012.
- van Houten CB, de Groot JA, Klein A, Srugo I, Chistyakov I, de Waal W, et al. A host-protein based assay to differentiate between bacterial and viral infections in preschool children (OPPORTUNITY): A double-blind, multicentre, validation study. Lancet Infect Dis. 2017 Apr 1;17(4):431-40.
- Srugo I, Klein A, Stein M, Golan-Shany O, Kerem N, Chistyakov I, et al. Validation of a novel assay to distinguish bacterial and viral infections. Pediatrics. 2017 Oct 1;140(4).
- Mor M, Paz M, Amir L, Levy I, Scheuerman O, Livni G, et al. Bacterial vs viral etiology of fever: A prospective study of a host score for supporting etiologic accuracy of emergency department physicians. PLoS One. 2023 Jan 30;18(1):e0281018.
- Papan C, Argentiero A, Porwoll M, Hakim U, Farinelli E, Testa I, et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: A prospective, multicentre cohort study. Clin Microbiol Infect. 2022 May 1;28(5):723-30.
- Halabi S, Shiber S, Paz M, Gottlieb TM, Barash E, Navon R, et al. Host test based on TRAIL, IP-10 and CRP for differentiating bacterial and viral respiratory tract infections in adults: Diagnostic accuracy study. Clin Microbiol Infect. 2023 Jun 1.
INTERPRETING MEMED BV RESULTS
What does an equivocal score mean?
A MeMed BV score within the equivocal range is a valid but etiologically non-informative test result. The physician is advised to proceed based on any other data available.
Compared to other biomarkers and clinical assessment tools (CRP, PCT and others), MeMed BV’s equivocal rate of 8-12%, is relatively low.
- MeMed data on file. Based on secondary endpoint analysis in Apollo Clinical Study (NCT04690569).
- Srugo I, Klein A, Stein M, Golan-Shany O, Kerem N, Chistyakov Iet al. Validation of a novel assay to distinguish bacterial and viral infections. Pediatrics. 2017 Oct 1;140(4).
Does a high bacterial numerical score reflect disease severity?
The MeMed BV score bin reflects the likelihood of a bacterial or viral infection and scores are not correlated with severity.
Does the accuracy increase during the course of an infection?
The MeMed BV score is robust across all stages of the infection for patients who have exhibited symptoms of acute infectious disease for less than or equal to 7 days and experienced fever within the last 7 days.
MEMED KEY LAB WORKFLOW
Q What is the MeMed Key® turnaround time?
The MeMed Key turnaround time is 15 minutes from a serum or venous whole blood sample.
*MeMed BV on venous whole blood is 510K cleared. Currently available only for the US market.
What Vacutainer should I use?
Collect a serum sample using a Serum Separator Tube (SST) or a whole blood sample using an EDTA (lavender top) tube, in accordance with best phlebotomy practices and local safety procedures.
*MeMed BV on venous whole blood is 510K cleared. Currently available only for the US market.
Can the MeMed Key® connect to an LIS system?
Yes, MeMed Key connectivity is currently supported by Data Innovations Instrument Manager™, Siemens POCcelerator™, and Orchard® Software. Our team is committed to ensure connectivity as required.
What are the dimensions of the MeMed Key®?
The MeMed Key is 13.4” L x 9.84” W x 9.84” H; 34cm L x 25cm W x 25cm L.

What is KeySyncTM and what are the benefits of connecting?
KeySync is a real-time, cloud-based support software program that tracks and analyzes trends and offers enhanced customer support for your lab at no additional charge.
Note that communication is unidirectional from the analyzer to the KeySync service. There is no inbound remote access to the MeMed Key®. Connected analyzers eliminate the need for USB data transfers between analyzer and other networked computers.
What type of security is built into the MeMed Key®?
The MeMed Key analyzer was designed with cybersecurity in mind and conforms to cybersecurity best practices. The MeMed Key uses a customized Linux version, and the operating system is hardened to prevent common types of exploits.
What are my options if we need more throughput than four tests an hour?
Because MeMed Key® is a relatively small analyzer – the size of a coffeemaker – many institutions opt for more than one MeMed Key.
Is MeMed BV® available on other analyzers?
Yes! The LIAISON® MeMed BV® test from DiaSorin is a revolutionary diagnostic solution that has received both CE and FDA clearance. It offers high throughput capabilities random access for STAT tests, and can be consolidated with a unique menu of specialty testing when run on the automated LIASON® family of analyzers. For more information call 1-800-328-1482.
Can we put the MeMed Key® in the Emergency Department or the STAT lab?
MeMed BV is a moderately-complex CLIA test run on the MeMed Key and can be placed anywhere that supports this workflow.
MEMED BV IMPLEMENTATION
How can I successfully implement MeMed BV® testing at my hospital?
MeMed offers both clinical and laboratory training per request. However, training is not a requirement for MeMed BV and MeMed Key operation.
Because every hospital is different, the Medical Science Liaison team is a resource to help develop a tailored MeMed BV educational program to meet your hospital’s specific clinical needs.
Our Technical & Customer Service team combines the latest technology, innovative training methods, and outstanding teaching expertise. They offer support, including but not limited to, training multiple shifts, assisting in the analytical verification process, go-live support, and more.
What kind of Lab support do you offer during implementation?
Our Applications team can be on-site to help with multiple aspects of implementation, including but not limited to: training multiple shifts, assisting in the analytical verification process, go-live support, and more.
What kind of support materials are available during implementation?
Relevant materials for both lab and clinicians including product IFUs, MeMed Key Quick Guide, custom clinical educational events & tools, protocol guidance, and more. Ask your Applications team for more information.
MEMED TECHNICAL SUPPORT
Where can I find the MeMed BV® and MeMed Key® Instructions for Use (IFU), Safety Data Sheets and other technical documentation?
You can find IFUs, Safety Data Sheets and other relevant product documents here. This is a password protected area for current customers. If you have any questions or need a password, please reach out to the Technical & Customer Service team.
Israel & Europe
Support Email: support@me-med.com
Phone Number: +972-(4) 8500302
United States
Support Email: help@me-med.com
Orders Email: USorders@me-med.com
Toll-Free Phone Number: 1-888-203-4550
Do you provide validation materials?
We do! We provide an analytical performance Verification Kit which contains serum samples spiked with recombinant TRAIL, IP-10 and CRP proteins. These samples are to be used for Accuracy, Precision, and Reportable Range as required by CLIA Guidelines (493.1253).
MEMED BV REIMBURSEMENT (USA only)
Is there a CPT code for MeMed BV®?
Yes, the AMA released CPT/PLA code 0351U effective October 1, 2022.
Is the reimbursement code priced on the Clinical Laboratory Fee Schedule CLFS?
Yes, MeMed BV® test (PLA code 0351U) is $260.50, effective Jan 1, 2023 on the CLFS.
Is there reimbursement coverage for your test?
Reimbursement coverage will vary depending on the payer and type of reimbursement. Please refer to your billing specialists or specific payer.

